I had the honor last month of conducting an intensive, three-day course on frozen desserts in the pastry kitchens at ICE, the latest in a long-running series known as the Center for Advanced Pastry Studies. The program - CAPS, for short - was initiated several years ago by pastry and baking instructor Michelle Tampakis, and the long list of illustrious pastry chefs who have brought their expertise to ICE in the past includes heavy hitters like Olivier Bajard, Laurent Branlard, Stephane Glacier, En-Ming Hsu, Michael Joy, Elisa Strauss, and Stephane Treand.

The CAPS program has become a unique and valuable resource not just for our students, faculty, and alumni, but also for the greater pastry community of New York City. As special guest instructors, the invited chefs are able to focus in on their respective specialties (cakes, candies, sugar, chocolate, plated desserts) over the span of some twenty hours of hands-on instruction to a small group of working chefs. It is this special format—and the opportunity to dive deep into a single subject—that I enjoy the most in my capacity as an instructor!
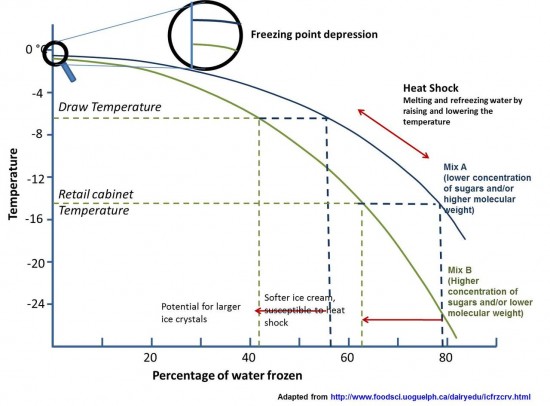
The theme for my course was frozen desserts, with a special focus on ice cream and sorbet. We jumped into things on the first day with an intensive look at the physics of ice cream and how sweeteners like sucrose, glucose, and lactose (the sugar present in milk) depress the freezing point of a liquid to different degrees. It is this very idea of freeze point depression that makes ice cream possible—a mixture that is soft enough to scoop when frozen, as opposed to a hard block of ice. The diagram above shows a range of temperatures and their effect on an ice cream. An interesting fact to consider: even at temperatures far below the freezing point, nearly 20% of the water in an ice cream base remains unfrozen!
Also crucial to understanding ice cream (and, in my opinion, all forms of cooking both sweet and savory) is identifying the composition and structure of our ingredients and how they function in any given recipe. After the brief primer on the physiochemical properties of frozen desserts, the class dove deeply into the composition of the various sugars, dairy, and egg products typically used in ice cream. The two charts below were heavily referenced during the class, especially when learning how to develop formulas for sorbets and ice creams with specific textures and flavors in mind.
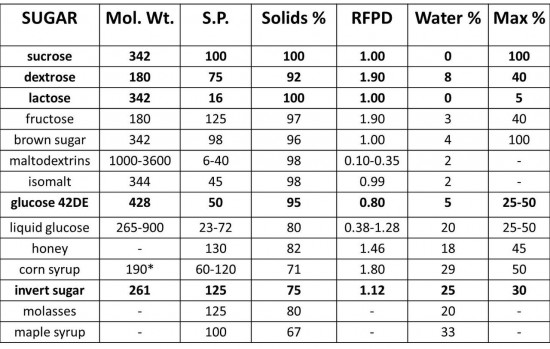
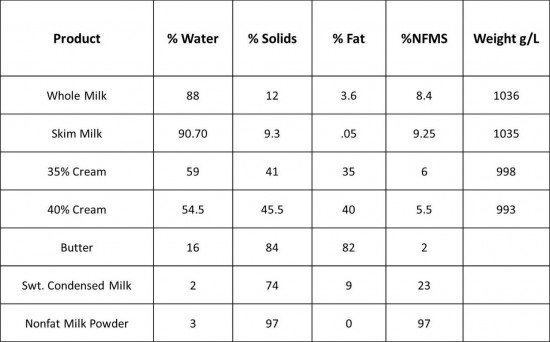
The ‘sugar’ chart offers detailed information such as water content and the degree to which each sweetener depresses the freezing point of water, as well as its relative sweetness, expressed in a numerical value. The dairy 'product' chart tells us how each form of milk, cream, or butter varies in water, fat, and solids content—all very important when formulating recipes.
With the close of the first day, the students prepared the most basic of frozen desserts, granités, a simple mixture of fruit juices or infusions frozen while agitated by hand. But on the second day, we got down to the serious business of formulating sorbet and ice cream recipes. The group produced over two dozen varieties, from simple flavors like chocolate and vanilla, to more complex and inventive concoctions, like balsamic vinegar, red pepper-mandarin, and cinnamon toast.
Beyond executing the basic recipes, we produced several versions of vanilla, with slight variations in fat content (from 6% to 14%), in order to evaluate how the different formulas produced different results in flavor, texture, and overrun. We also compared the effects of churning the ice creams in a conventional freezer versus a PacoJet, which is increasingly common in small restaurant kitchens. And after tasting our range of vanilla bases, we also put them through a melt-down test to compare how methods and recipes affect the rate at which each ice cream melted.
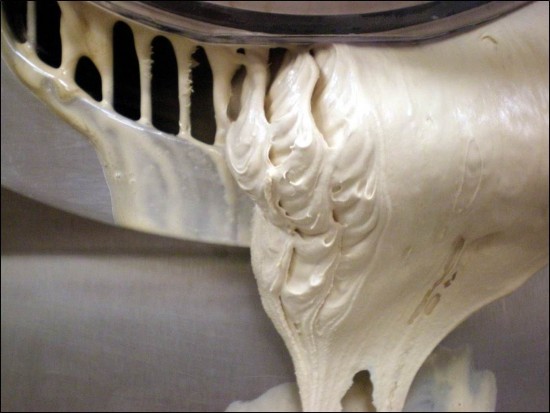
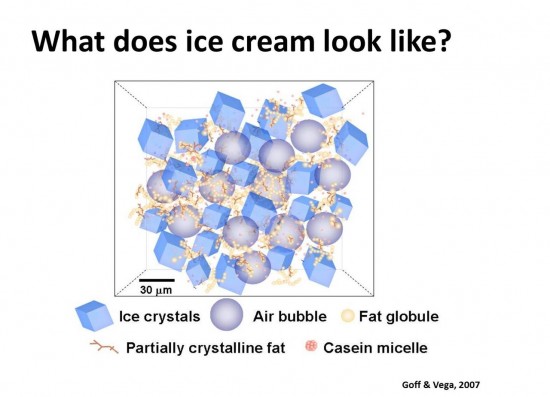
The third and final day gave us the opportunity to discuss some of the applications of our efforts, including fun novelty dessert items like frozen bars and ice cream sandwiches. We also spent a few moments exploring the micro-structure of ice cream, visualizing frozen desserts on a near-molecular level to better understand its behavior both before and after freezing. The diagram below shows the delicate structure of air bubbles (ice cream is, technically, a kind of foam), coalesced milk fat, and solid ice crystals, all dispersed within the unfrozen solution of water and sugars.
From my perspective, each of the students—whose experience ran from working pastry chefs, to enthusiastic amateurs, to artisanal ice cream makers—took something away from the class that they could apply in their own unique environment.
As for myself, all of the prior research that went into the materials certainly expanded my own knowledge of frozen desserts, and I’m hoping to make this three-day seminar a recurring feature of the CAPS program here at ICE!




