When I began training bartenders nearly two decades ago, I would open with the question, "Did you ever feel like someone somewhere gave a class on how to act in a bar and you weren't invited?"
Even among that group of service professionals, the answer was always a sea of uncomfortable giggles and nods. Since then, both the beverage industry and beverage consumers have grown exponentially in their understanding of the history and science behind cocktails and spirits, but I still start my classes with the same question and the response hasn't changed.
If anything, bar culture has become more intimidating, drinks more complex and the perception of being an outsider has increased among the average consumer. So the purpose of this blog series will be the same as that of my very first class: to demystify the bar.
So we’ll begin at the beginning, with the seemingly simplest and most basic questions I've encountered — many of which actually are neither simple nor basic. If you're new to either side of the bar, you'll gain a solid understanding of the products, techniques, tools and terminology we use. If you're a veteran mixologist, I'll try to throw in enough science and history to keep you interested and hopefully provide additional context for what you've already mastered.
In either case, please send in any questions you have, and be assured that if you have a question, someone else has the same question, so don't be shy.
That said, let's start with the most basic bar question of all.
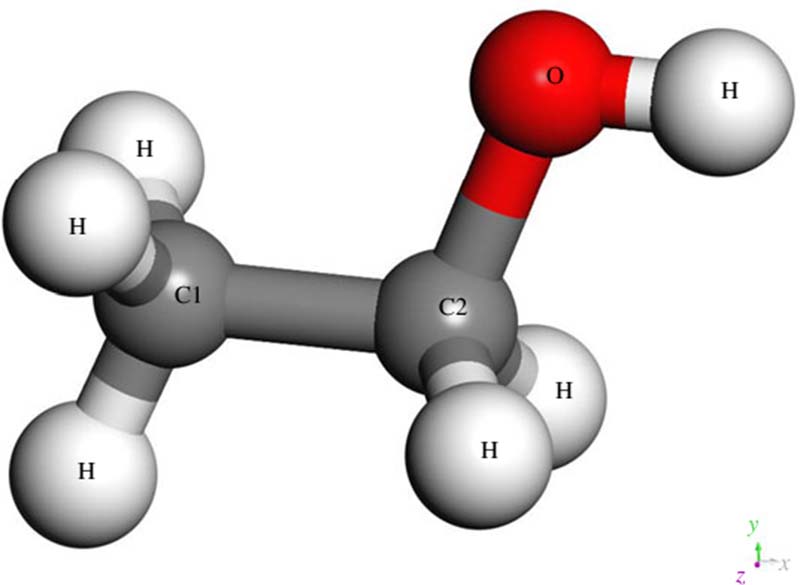
What is alcohol?
Not a simple question in the least, as it turns out, because at the bar we use the very broad term "alcohol" to refer to just one of many chemicals classified as alcohols: ethyl alcohol, or ethanol. There are many different alcohols besides ethyl, including amyl alcohol, butyl alcohol, methyl alcohol and isopropyl alcohol. They are all organic compounds distinguished by an oxygen-hydrogen functional group hanging off the base molecule, so they all have a similar chemical formula and share similar properties:
- Alcohols are generally colorless (the shade of dark spirits like whiskey comes from aging in casks, which we'll be covering in a future article).
- Most are flammable (the ethanol in vodka is exactly the same as the ethanol in the gasoline in your car).
- They are solvents (you've probably used isopropyl alcohol, or isopropanol, to remove sticker residue).
- They are toxic in varying dosages.
The only alcohol we drink, though, is ethyl alcohol (CH3CH2OH) which is the least toxic of the family. The word "intoxicated" refers to this unpleasant trait, and in relatively small quantities (about 0.55 percent blood alcohol content) ethanol can be just as deadly to us as its relatives. Ethyl alcohol also kills microorganisms quite handily, which is why it's the active ingredient in hand sanitizer. In fact, during the pandemic shutdown, many distilleries survived by simply putting the ethanol they were producing into sanitizer bottles instead of liquor bottles.
More Like This: Cocktail Rules to Live By
In lower dosages, ethanol also has psychoactive effects, which we call getting drunk. This is the primary reason many consumers today drink alcoholic beverages, though it turns out we were actually consuming alcohol for its toxicity to microorganisms much earlier, and long before that because of its caloric value. But that's also a subject for an upcoming article.
Ethanol is the only active ingredient in spirits like vodka, gin, rum, Tequila and whiskey, and they all contain about the same amount: usually 40 percent alcohol by volume (ABV) or 80 proof. Some spirits are bottled at higher ABVs, but usually by only a few percentage points. So it doesn't matter what spirit you're drinking, if the volume of liquor in your glass is the same, you're consuming the same amount of the exact same chemical — any differences are down to flavor and sometimes color.
Students are often surprised by this, and ask me why they get drunker on certain spirits (like Tequila) than others (like gin). I'll then ask when and how they drink each, and on what occasions. Common answers like "on vacation,” “as a shot" and "night out partying" for Tequila versus "after work,” “in a gin and tonic" and "happy hour" for gin explain the mystery. The difference isn't the spirit in the drink, it's the spirit of the drinker.
To take a deeper dive into spirits like Scotch, Tequila and rum, join me in one of my upcoming recreational classes at ICE. Click here to register.
Anthony Caporale is ICE's Director of Spirits Education, and consults for some of the largest beverage companies in the world. He also created The Imbible series of hit NYC musicals about the history of cocktails and spirits. Learn more at anthonycaporale.com.




