One of the many hats I wear as creative director is that of a guest lecturer. Perhaps my favorite task at ICE is leading our professional Pastry & Baking students' introduction to dairy products during the very first days of their program.
If you know me, then you know that I have become a bit of a dairy nerd, always looking to better understand the composition, structure, and function of milk and all of its derivatives. And while I present a great deal of technical information during my dairy lecture—in addition to offering a tasting of some two dozen products—my goal is simply to present this humble, common ingredient in a new light. Retaining every specific fact and figure isn’t as important as getting our students to start thinking about all ingredients in an analytical way—in other words, what they bring to a recipe and how they affect its final outcome.

I like to say that as pastry chefs, we’re often required to "predict the future." As opposed to a savory cook, who might be able to taste and adjust a preparation over the course of the entire process, a pastry cook typically must combine carefully measured ingredients in advance, without the ability to make adjustments once the cooking process begins. Thus, a technical and scientific understanding becomes a crucial part of what we do. I typically begin my dairy lecture by challenging the students to think small, to shrink themselves down to tiny microscopic particles, and to look at milk as a sum of many different parts.
As we usually take milk for granted, I break down its composition: roughly 88% water, 3.5% fat and 8.5% nonfat milk solids, mostly made up of proteins and lactose. I further describe how these components are arranged. Not only does the composition of any dairy product affect its attributes, but so can that arrangement of water, fat and nonfat solids. We can actually describe the structure of milk in three ways: an emulsion, a suspension, and a solution.
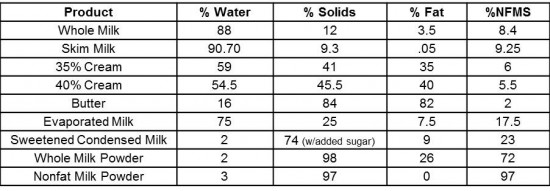
As the students taste their way through a range of liquid milk products from skim milk to heavy cream, I shift the conversation to whipped cream. In real time, I whip cream through various stages to explain what is happening to the internal structure—how the milk fat starts to form a sort of scaffolding around the air that is incorporated during the whipping process. By understanding how the milk fat coalesces, the students learn what changes take place as we continue to whip and when we’ve reached the ideal consistency (which is much softer than one would think!).

As we take the cream from soft to firm peaks and beyond, we continue the whipping until we’ve made butter. This is usually the point at which I notice something "click" amongst the class, as they begin to better understand the relationship between one dairy product and another. In addition to our bespoke butter, we taste a handful of commercial examples and begin to discuss the role butter plays in various doughs (and why a mere 1% difference in fat content can make a significant difference, especially when preparing laminated dough). The increased plasticity that comes with a higher milk fat content is important for such doughs that require hundreds of fine layers of dough and butter. This is why the "default" butter we use at ICE is high quality European-style butter with a fat content of 83%.

While discussing the by-product of our butter—the protein- and lactose-rich buttermilk—we touch upon the complex world of cultured milk products, from yogurt and sour cream, to soft and hard cheeses. The focus here turns to the milk proteins, and a basic understanding of how they are denatured, or irreversibly transformed, by heat, acids and enzymes to turn what once was liquid milk into a wide variety of textures. By tasting samples such as cream cheese, mozzarella and gruyère, we briefly explore how different milk products, cultures and processing steps determine the myriad flavors and characteristics that make each cheese unique.
I wrap up the lecture with a look at the processes and reactions that occur when we cook dairy products, most important of which are the Maillard reactions, which result in appealing brown colors and complex flavors that we associate with virtually all cooked foods. As I begin to melt and brown some of the butter we made in class, I define and differentiate caramelization and Maillard reactions. Caramelization simply refers to the point at which sugar actually begins to decompose (about 325˚F).
Lucky for us, decomposing sugar tastes wonderful, and is the base for countless pastry preparations! Maillard reactions, however, occur when sugars (glucose, fructose, and lactose) and proteins are heated together; after enough moisture is removed (and at far lower temperatures than true caramelization), characteristic browning and the creation of new flavor compounds begin to emerge. Understanding this process on even a basic level gives a cook much more insight into what is happening in the pan or in the oven as we cook.

I close the two-hour lecture by applying these Maillard reactions (named after the French chemist who discovered them a mere 100 years ago) to products like dulce de leche and the notion of ‘roasted’ white chocolate. Though counter-intuitive at first glance, the practice of carefully roasting white chocolate to create more complex flavors has, in recent years, become an established technique.
This creative (and delicious) idea would have likely never happened were it not for a few pastry chefs who understood the science that makes it possible. Not only does such intimate knowledge of our ingredients make us better cooks, but eventually that knowledge can lead to new creative developments. In addition to the dairy lectures I give to our incoming pastry students, I also offer a more in-depth dairy class, Composition and Structure: The Humble Complexity of Milk. The next session is Thursday, September 11th, from 6:00 to 10:00 pm. I hope to see you there!




